Introducción
 Desde hace años se utilizan corticoides potentes, activos cuando se administran por vía tópica y con actividad sistémica mínima para controlar el asma y la rinitis alérgica cuando se administran en las dosis recomendadas. Provocan menos efectos adversos que los corticoides administrados por vía sistémica.
Desde hace años se utilizan corticoides potentes, activos cuando se administran por vía tópica y con actividad sistémica mínima para controlar el asma y la rinitis alérgica cuando se administran en las dosis recomendadas. Provocan menos efectos adversos que los corticoides administrados por vía sistémica.
Sin embargo, los resultados de distintas investigaciones muestran que algunos niños asmáticos que recibieron budesonida y dipropionato de beclometasona presentaron una disminución en su crecimiento lineal.
La Food and Drug Administration (FDA) de los Estados Unidos determinó que los estudios sobre el crecimiento eran un factor importante para determinar la seguridad y los efectos de los tratamientos con corticoides administrados por vía inhalatoria a nivel sistémico, y tenían mayor sensibilidad que las pruebas estandarizadas de evaluación del eje hipotálamo hipofisario adrenal. Recomiendan que los laboratorios farmacéuticos incluyan la evaluación del crecimiento como parte de los programas de desarrollo clínico, con indicaciones específicas en la implementación de dichos estudios.
Efectos sobre el crecimiento
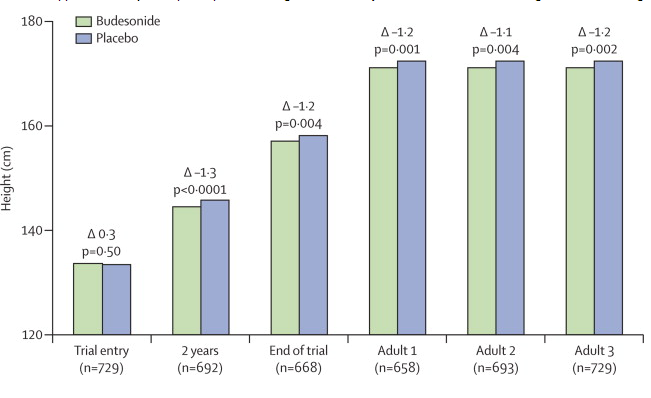
Las investigaciones realizadas con períodos de seguimiento de 3 a 6 años sugieren que la mayor reducción en la velocidad de crecimiento ocurre en los primeros 6 meses de tratamiento. Pasados 2 años aproximadamente, la velocidad de crecimiento es similar a la que presentan los niños que reciben placebo. En consecuencia, los mayores efectos sobre la estatura son más frecuentes en los primeros 2 años de tratamiento que en los años posteriores.
En la mayoría de los estudios que compararon la estatura alcanzada por individuos que habían recibido corticoides por vía inhalatoria cuando eran niños debido al asma, con otros sujetos asmáticos que no recibieron dicho tratamiento y controles no asmáticos, no se encontraron diferencias en la estatura. Sin embargo, en un estudio se informó sobre una diferencia significativa en la estatura alcanzada.
En una cohorte con seguimiento prolongado, perteneciente al estudio The Childhood Asthma Managemet Program (CAMP), se comprobó que las estaturas en la adultez del grupo tratado con budesonida fueron 1.2 cm menores que en el grupo placebo, un dato similar a los resultados obtenidos al finalizar el estudio original. Dicho análisis fue el primer seguimiento realizado hasta la edad adulta mediante un estudio aleatorizado, y permitió analizar el efecto del tratamiento continuo con corticoides por vía inhalatoria sobre el nivel de la estatura adulta.
Los efectos de los corticoides inhalados sobre el crecimiento parecen ser dependientes tanto de la dosis como de la duración del tratamiento. La intensidad de los efectos sistémicos depende de las propiedades farmacocinéticas (absorción, distribución y eliminación del fármaco), mientras que la dosis eficaz realizada depende del sistema y de la técnica de administración y potencia de la molécula utilizada.
Los nuevos corticoides, como el propionato de fluticasona, el furoato de mometasona y la ciclesonida, tienen una biodisponibilidad oral menor (< 1%) y mayor lipofilia. La razón entre la actividad terapéutica y la sistémica indica un mejor índice terapéutico.
Se encuentran en investigación las acciones que ocurren a nivel del epitelio respiratorio cuando finalmente se deposita el fármaco, los efectos de las interacciones entre la dosis final y el tejido pulmonar y cómo difieren los mecanismos de acción a nivel celular según las distintas formulaciones.
Se ha observado que el efecto sobre el crecimiento es dependiente de la dosis, y que si cualquier corticoide por vía inhalatoria se administra en dosis suficientemente altas, la velocidad de crecimiento se reducirá. Con la disminución de la dosis, se recupera la velocidad de crecimiento, pero los resultados finales son variables. Uno de los estudios realizados mostró que, si bien el tratamiento durante 4 meses no producía una disminución de la estatura, ésta sí se observaba con 7 meses de tratamiento.
La línea de velocidad de crecimiento en los niños depende de la edad, la etapa puberal, la genética y la nutrición. Las curvas de crecimiento normal presentan 3 fases: desde el nacimiento hasta los 2 años depende en general de la nutrición; en la segunda fase, que se extiende aproximadamente hasta el final de la primera década, el crecimiento depende de la hormona de crecimiento y el estado nutricional. La última fase, de empuje puberal, se desarrolla a lo largo de la segunda década de la vida. Además de ser regulada por factores genéticos y nutricionales, es consecuencia del estímulo de las hormonas sexuales sobre la secreción de la hormona de crecimiento.
En la actualidad, faltan investigaciones acerca del efecto sobre la estatura con el tratamiento corticoideo por vía inhalatoria en los adolescentes. También, es importante considerar el efecto de la enfermedad asmática y la rinitis crónica sobre el crecimiento y desarrollo de los niños y adolescentes.
Efectos sobre la densidad mineral ósea
Los efectos de los corticoides sobre la densidad mineral ósea en los niños parecen ser más susceptibles de intervención. Los tratamientos a largo plazo con corticoides inhalados mostraron ser más seguros que las exposiciones frecuentes a los corticoides por vía oral sobre la acumulación mineral ósea.
Se destaca que una nutrición adecuada, con la ingesta suficiente de calcio y vitamina D, debería prevenir o mitigar los efectos de los corticoides sobre la densidad mineral ósea. También, demostró ser útil la indicación de una rutina de ejercicios de carga acorde con la edad y condición física.
Los posibles efectos adversos de los corticoides por vía inhalatoria deben sopesarse frente a los importantes y bien establecidos beneficios de estos fármacos para controlar el asma persistente. Para reducir al mínimo los efectos adversos, el tratamiento con corticoides inhalados siempre debe tratar de alcanzar la menor dosis eficaz que logre controlar la enfermedad.
Conclusiones
A partir de las nuevas pruebas científicas reunidas en los últimos 7 años, se conocen mejor los beneficios y riesgos del tratamiento prolongado con corticoides inhalados en los niños con asma o rinitis alérgica, en especial los riesgos de supresión del crecimiento y disminución de las densidad mineral ósea. Cada uno de estos riesgos es mediado por mecanismos diferentes, por lo que se requieren distintos enfoques para minimizarlos. La falta de crecimiento detectada se relaciona con tratamientos prolongados, mientras que la disminución de la densidad ósea se vincula con tratamientos más breves y repetidos.
Cuando se detecta una disminución del crecimiento en un paciente que ha recibido tratamiento de largo plazo con dosis elevadas de corticoides inhalados durante 7 a 12 meses, probablemente se verá afectada su estatura final.
Para minimizar los efectos secundarios, se deben reducir las dosis hasta alcanzar la dosis mínima eficaz para el control de los síntomas asmáticos. Puede considerarse el agregado de broncodilatadores de acción prolongada y el uso de formulaciones de corticoides inhalados con un alto índice terapéutico y pocos efectos sobre el crecimiento.
En relación con el tratamiento intermitente con corticoides inhalados, aún se desconoce su impacto en el largo plazo (> 1 año), sobre el desarrollo o la declinación de la función pulmonar.
Los efectos de los corticoides sobre la densidad mineral ósea pueden prevenirse o mitigarse. Se recomienda utilizar dosis eficaces mínimas, y asegurar una nutrición adecuada, que incluya calcio y vitamina D. También, resulta positivo el agregado de una rutina de ejercicios con carga.
Frente al riesgo de posibles efectos adversos de los corticoides inhalados, deben considerarse los numerosos y comprobados efectos beneficiosos de estos fármacos para controlar el asma persistente.
*SIIC: Sociedad Iberoamericana de Información Científica