![]()
| Abreviaturas EH encefalopatía hepática EHM encefalopatía hepática mínima EHA encefalopatía hepática asintomática EHS encefalopatía hepática sintomática AACR Aminoácidos con cadena ramificada LOLA L-ornitina-L-aspartato MARS Sistema de recirculación de adsorbentes moleculares (por las siglas del inglés (Molecular Adsorbent Recirculating System) TE Tratamiento estándar |
Introducción
La encefalopatía hepática (EH) es un síndrome neuropsiquiátrico que se produce con frecuencia en la cirrosis descompensada. El cuadro clínico oscila desde síntomas clínicamente imperceptibles en la EH mínima (EHM), que sólo se detecta con pruebas neuropsicométricas, hasta el coma en los casos más graves.
Según la nomenclatura de 1998 del Working Party for Hepatic Encephalopathy para la EH:
- Tipo A es la EH secundaria a la insuficiencia hepática aguda.
- Tipo B es la hiperamoniemia entérica (sin enfermedad hepática).
- Tipo C se asocia con enfermedad hepática crónica.
La gravedad de la EH se clasifica según los criterios de West Haven (grados 1-4), pero hay también otras terminologías. En el nuevo léxico, llamado SONIC (espectro de deterioro neurocognitivo en la cirrosis), la EH asintomática (EHA) incluye la EHM y la EH grado 1. La EH sintomática (EHS) incluye los grados 2 a 4 de EH (Tabla)
Tabla. Grados de deterioro en la encefalopatía hepática
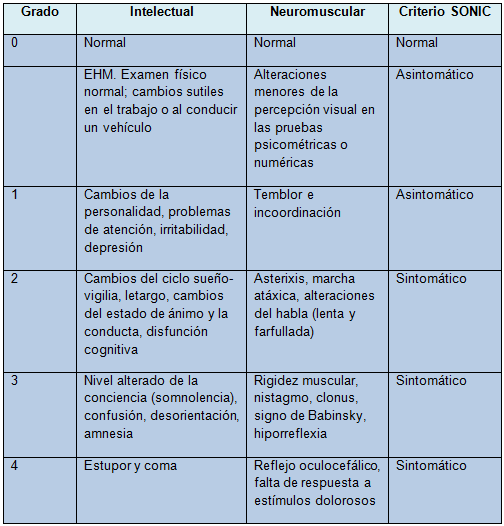
EHM: encefalopatía hepática mínima; SONIC: espectro de deterioro neurocognitivo en la cirrosis.
La EH episódica aparece durante períodos breves y puede fluctuar en su gravedad, mientras que la EH persistente altera la función ejecutiva cotidiana. La mayoría de los pacientes con EHS episódica (grado 2 o mayor) necesitarán hospitalización.
La EH se produce a la larga en hasta el 50% de los pacientes con cirrosis y se debe considerar el trasplante hepático en estos casos. Su tratamiento continúa siendo un tema importante de investigación. Los disacáridos no absorbibles (eg, lactulosa y lactitol) y los antibióticos no absorbibles (eg, neomicina y rifaximina) constituyen los pilares del tratamiento.
Hasta el 80% de los episodios de EHS se desencadenan por problemas tales como infección o hemorragia gastrointestinal. El objetivo del tratamiento del paciente hospitalizado con EHS es corregir el desencadenante subyacente y reducir la producción de amoníaco mediante fármacos.
La mayoría de los pacientes necesitan tratamiento de mantenimiento al alta del hospital, como profilaxis secundaria de la EHS episódica, pero los datos disponibles sugieren que muchos de ellos no los reciben.
El trabajo de Volk et al menciona una gran proporción de rehospitalización (69%) en una cohorte de pacientes con cirrosis descompensada (n = 402). Uno de los motivos más frecuentes y prevenibles de la rehospitalización fue la EH recidivante debido a la falta de información del paciente o al empleo inapropiado de la lactulosa. Es necesario asegurarse que los pacientes reciben y están informados y educados sobre el tratamiento de mantenimiento para la prevención secundaria de la EHS al alta del hospital.
En este trabajo, que se basa sobre una búsqueda bibliográfica en PubMed de los estudios publicados entre 2003 y 2013, se resume la evidencia sobre los tratamientos médicos óptimos, pero no se trata el tema del trasplante hepático.
Evidencia sobre los tratamientos de inducción para la ehs episódica
Disacáridos no absorbibles
La lactulosa (b-galactosidofructosa) y el lactitol (b-galactosidosorbitol) disminuyen las concentraciones de amoníaco por:
1) acidificación del colon, con conversión del amoníaco a amonio, cambiando la flora colónica de bacterias que producen ureasa a las que no la producen.
2) efecto laxante.
Los estudios clínicos muestran que los disacáridos no absorbibles son de eficacia variable. Un metanálisis de 2004 halló que fueron superiores al placebo, pero no mejoraron la supervivencia. Cuando se incluyeron sólo los estudios de alta calidad en este metanálisis, los disacáridos no absorbibles no tuvieron efecto sobre la EH. A pesar de estos resultados, la lactulosa sigue siendo el tratamiento de primera línea para la EHS episódica aguda. Décadas de experiencia clínica con lactulosa indican su eficacia para la EHS episódica, salvo en los casos más graves. La discordancia entre la eficacia en estudios clínicos y en la vida real responde a diversas causas, entre ellas la heterogeneidad en los tipos de EH (mínima vs sintomática vs crónica), las diferencias en la importancia de los desencadenantes de la EH y la subjetividad de las herramientas para evaluar la EH. Las recomendaciones clínicas aconsejan la lactulosa o el lactitol como tratamiento de primera línea.
Neomicina, metronidazol y otros antibióticos
La neomicina es un aminoglucósido de escasa absorción empleado para disminuir el amoníaco derivado de las bacterias intestinales. Está autorizada por la Food and Drug Administration (FDA) para la EHS episódica, pero no para la EH crónica. Numerosos estudios de años atrás exploraron la eficacia de estos antibióticos en la EH. En general, la evidencia a favor de la neomicina en la EHS episódica es débil y su empleo se complica debido al riesgo de efectos tóxicos sobre la audición y el riñón. Otros estudios pequeños evaluaron el metronidazol y la vancomicina, pero el riesgo de efectos tóxicos neurológicos y de colonización por enterococos resistentes a vancomicina, respectivamente, es mayor que sus posibles beneficios.
Rifaximina
La rifaximina no está autorizada por la FDA para el tratamiento de la EH sintomática episódica, sino sólo para la prevención secundaria de la EHS. En este trabajo se mencionan estudios de la década de 1990: varios de rifaximina en relación con lactitol, otro de rifaximina vs placebo, otro de rifaximina en relación con lactulosa. Dado el pequeño número de estudios y sus defectos metodológicos, aún no se sabe a ciencia cierta la utilidad de la rifaximina como monoterapia para la EHS episódica.
A pesar de esta falta de evidencia previa, el empleo de rifaximina para la EHS episódica junto con lactulosa es cada vez más frecuente. Un estudio aleatorizado controlado reciente (n = 120) efectuado por Sharma et al comparó la rifaximina y la lactulosa con lactulosa y placebo en pacientes con EHS. El 80% de los pacientes padecían EH grave, grado 3 o 4 y el 70% eran clase C de Child-Turcotte-Pugh (Child-Turcotte-Pugh es una escala para la clasificación pronóstica de la hepatopatía. El resto eran clase B de la clasificación de Child-Turcotte-Pugh.
Los pacientes del grupo lactulosa y rifaximina tuvieron mayor proporción de mejoría total de la EH (76% vs 50,8%, P < 0,004), menor duración de la hospitalización y gran mejoría de la mortalidad a 10 días (49,1% vs 23,8%, P < 0,05). La tasa de mortalidad muy alta en la rama lactulosa más placebo genera preocupación sobre la validez de este estudio, que se debería repetir con más pacientes en múltiples instituciones. Mientras tanto, estos son los mejores datos disponibles para tomar decisiones terapéuticas basadas en la evidencia.
Zinc
Ambas vías de la reducción del amoníaco (la hepática y la del músculo esquelético) se alteran con la deficiencia de zinc. Cuatro estudios aleatorizados controlados se ocuparon del tratamiento con zinc, con resultados heterogéneos.
El estudio más reciente, de Takuma et al en 2010, aleatorizó a los pacientes con cirrosis y grado 1 y 2 de EH resistentes al tratamiento habitual a recibir tratamiento con zinc (n = 39) además de lactulosa y aminoácidos con cadena ramificada (AACR) vs no zinc (n = 40) con AACR y lactulosa. A los 6 meses la EH mejoró en 21 (54%) vs 10 (26%) de los pacientes en las ramas zinc vs no zinc, con 16 pacientes tratados con zinc (41%) que mejoraron a EH grado 0. Aunque con algunos defectos, éste es el único estudio reciente con evidencia razonable que sugiere el beneficio del zinc. Éste se tolera relativamente bien, con raros efectos adversos de dispepsia y deficiencia de cobre (con su empleo prolongado a dosis altas).No hay datos suficientes para definir la dosis óptima.
L-Ornitina-L-Aspartato
La L-ornitina-L-aspartato (LOLA) es una sal compuesta que estimula a la ornitina transcarbamoliasa y la carbamoil fosfato sintetasa y es un sustrato para la formación de urea. También estimula la síntesis de glutamina en el músculo esquelético y por lo tanto disminuye el amoníaco.
Se efectuaron dos estudios aleatorizados, controlados con placebo, doble ciego en Alemania, con el empleo de formas intravenosas y orales de LOLA en pacientes con EH crónica y en ambos mejoraron las pruebas de conexión, los valores del amoníaco y los parámetros de la EH (el estado mental y el índice de encefalopatía portosistémica).
La EH episódica recidivante se excluyó de estos estudios. Hay pocos datos sobre el tratamiento o la profilaxis de la EHS episódica. Un estudio de Pakistán evaluó la LOLA como tratamiento complementario vs placebo en pacientes que recibían el tratamiento estándar (TE). Los pacientes con EH grado 2 o más mejoraron significativamente en el grado de EH con el TE + LOLA (79%) vs TE + placebo (55%) (P= 0,019).
Aminoácidos con cadena ramificada
Los aminoácidos plasmáticos están alterados en los pacientes con cirrosis, con disminución de los AACR y aumento de los aminoácidos aromáticos. Los AACR son una fuente de glutamato, que contribuye a metabolizar el amoníaco en el músculo esquelético. El aporte complementario de AACR puede mejorar la síntesis de albúmina, disminuir la resistencia a la insulina, disminuir el carcinoma hepatocelular y mejorar la función inmunitaria.
En dos estudios aleatorizados controlados los AACR mejoraron, en uno de ellos los criterios de valoración compuestos de muerte/hospitalización y en el otro la insuficiencia hepática, la hemorragia por várices, el carcinoma hepatocelular y la mortalidad. Los AACR se estudiaron asimismo en la EH.
En un estudio reciente, se administraron AACR a 58 pacientes con un episodio previo de EHS y se los comparó con 58 pacientes que recibieron maltodextrina. No hubo diferencia significativa en la frecuencia de EH recidivante, pero los resultados se deben interpretar con cautela porque muchos pacientes se perdieron para el seguimiento.
En la actualidad, la European Society for Clinical Nutrition and Metabolism recomienda el empleo de 1,2 g/kg por día de proteína para la cirrosis compensada y 1,5 g/kg por día para la cirrosis descompensada. Esta recomendación se basó sobre los resultados de un estudio aleatorizado controlado de una dieta proteica normal (1,2 g/kg por día) vs una dieta restringida, que no tuvo efecto sobre la evolución de la EH episódica, pero aumentó el catabolismo muscular en el grupo de proteínas bajas.
La European Society for Clinical Nutrition and Metabolism proporciona también una recomendación grado A para el aporte complementario estándar de proteínas en pacientes con EH grado 2 o menos y preparados de AACR para la EH grados 3 y 4.
Embolización percutánea de grandes shunts portosistémicos
Se publicaron dos grandes series retrospectivas sobre la eficacia y la seguridad de la embolización de grandes shunts portosistémicos en pacientes con EH resistente al tratamiento médico. En un estudio multicéntrico europeo (n = 37), el 59% de los pacientes estaban sin EH dentro de los 100 días y el 48% estaban sin EH en un promedio de 2 años después de la embolización.
La seguridad a largo plazo fue buena, sin complicaciones. En la serie más grande de los EEUU (n = 15), el 90% de los pacientes con cirrosis mejoraron a los 2 meses del procedimiento, sin complicaciones significativas. La mediana de la puntuación MELD (MELD es un sistema de puntuación para medir la gravedad de la enfermedad hepática crónica), en ambos estudios fue 13. La regresión logística efectuada en el estudio europeo sugirió que los pacientes con puntuación MELD mayor de 11 tenían riesgo de recidiva de EH.
Sistema de recirculación de adsorbentes moleculares
El sistema de diálisis de toxinas hepáticas mediante la recirculación de adsorbentes moleculares (Molecular Adsorbent Recirculating System, MARS) se creó en 1999 para eliminar las toxinas ligadas a las proteínas y a la albúmina, como la bilirrubina, los ácidos biliares, el óxido nitroso, entre otros y también elimina el amoníaco no ligado a las proteínas, que se acumula en la insuficiencia hepática.
Aunque aún se cuestiona el efecto del MARS sobre la supervivencia de los pacientes con insuficiencia hepática, tres estudios informaron mejoría de la EH. El más reciente (RELIEF) incorporó a 189 pacientes y evaluó MARS más TE vs TE solo con los criterios de valoración de supervivencia sin trasplante a 28-90 días. Estos criterios no se cumplieron, pero se demostró la seguridad del procedimiento.
La proporción de pacientes con EH grado 3 o 4 que mejoraron a EH grado 0 o 1 fue mayor en los pacientes tratados con MARS (62,5%) que con TE (38,2%).En otro estudio efectuado para evaluar el efecto de MARS sobre la EH, se aleatorizó a 70 pacientes con EH grado 3 y grado 4 HE para recibir MARS + TE vs TE solo. Una mayor proporción de pacientes tuvo mejoría de 2 grados en la EH en la rama MARS (media, 34%) vs la rama TE (19%).
En el estudio más pequeño, el MARS tuvo una mejoría estadísticamente significativa de la EH en 9 pacientes con hepatitis alcohólica y EH (sin TE).La FDA aprobó el empleo de MARS para la EH relacionada con la descompensación de la hepatopatía crónica. Aunque el MARS puede disminuir la biodisponibilidad de ciertos antibióticos, parece ser una opción viable para pacientes con EH grave que no responde al TE.
Evidencia sobre las estrategias de prevención secundaria y de mantenimiento para la EH
Lactulosa
Los resultados de dos estudios recientes proporcionaron evidencia sólida para emplear lactulosa sola o lactulosa y rifaximina para la prevención secundaria.
Sharma et al efectuaron un estudio abierto y aleatorizaron a 140 pacientes para recibir placebo o lactulosa tras su recuperación de un episodio de EHS. El 19,7% de los pacientes tratados con lactulosa-(12 de 61) sufrieron EHS recidivante vs el 46,9% (30 de 64) en la rama placebo durante una mediana de seguimiento de 14 meses. En el trabajo de Agrawal et al, los pacientes recuperados de EHS recibieron lactulosa, probióticos o ningún tratamiento. Los resultados en la población con intención de tratar demostraron una tasa de EHS significativamente menor para la lactulosa (37,5%) y los probióticos (45,4%) en relación con ningún tratamiento (64,1%).
Rifaximina
En 2010, Bass et al publicaron los resultados de un estudio aleatorizado controlado de rifaximina (n = 140) vs placebo (n = 159) para la prevención secundaria de la EHS episódica en pacientes con 2 o más episodios previos, que estaban en remisión cuando se incorporaron al estudio. Más del 90% de los pacientes de ambas ramas estaban tomando lactulosa.
Los pacientes del grupo rifaximina tuvieron una reducción de las recidivas de EH (31 de 140) mayor que los del grupo placebo (73 de 159). También disminuyeron en un 50% las hospitalizaciones en el grupo rifaximina (19 de 140) en relación con las del grupo placebo (36 de 159).
Los episodios adversos fueron similares en ambos grupos. Sobre la base de los datos de este estudio de fase 3, la FDA aprobó la rifaximina para la prevención secundaria de la EHS. Hay un estudio en marcha sobre la seguridad y la eficacia de la rifaximina en pacientes con cirrosis con puntuaciones MELD de 25 o más y otro sobre rifaximina más lactulosa vs rifaximina sola para la prevención secundaria.
Los pacientes con puntuaciones MELD altas o cirrosis clase B/C de Child-Turcotte-Pugh necesitan profilaxis secundaria tras su recuperación de EH episódica espontánea o desencadenada. Para los pacientes con cirrosis clase A de Child-Turcotte-Pugh que están compensados, pero sufren una EHS aislada en el contexto de una infección o una hemorragia gastrointestinal, las decisiones sobre la profilaxis secundaria se deben tomar caso por caso. Idealmente, se les deben efectuar pruebas neuropsicométricas para EH asintomática algunas semanas después del alta del hospital. No obstante, estas pruebas no son prácticas y sería muy necesaria una herramienta clínica sencilla para detectar la EH asintomática.
Inducción y tratamiento de mantenimiento de la EH
Inducción y tratamiento de mantenimiento para el primer episodio de EHS episódica, grados 1 y2 de West Haven
El primer paso en el tratamiento de la EHS episódica es la evaluación de los desencadenantes típicos, entre ellos hemorragia gastrointestinal, infecciones, nuevos medicamentos (como opioides o benzodiacepinas), estreñimiento, diarrea, deshidratación, alcalosis o hipopotasiemia e hipoxemia.
Cuando se encuentra un desencadenante - hasta el 80% de los pacientes lo tienen- se recomienda tratarlo, al mismo tiempo que se administra tratamiento con lactulosa. Para los que no responden al tratamiento inicial, es importante revaluar el diagnóstico de EHS, considerar la posibilidad de otros desencadenantes y asegurarse que el paciente tenga 3 o 4 deposiciones por día mientras reciba lactulosa.
Por ejemplo, puede ser necesaria una tomografía computarizada (TC) craneal cuando al revaluar al paciente se encuentra una alteración neurológica focal nueva. Si el diagnóstico de EHS es correcto, no se hallan otros desencadenantes y las deposiciones son adecuadas, se puede agregar neomicina o rifaximina al tratamiento con lactulosa.
La neomicina está autorizada por la FDA para esta indicación, a pesar de que preocupan sus efectos tóxicos renales y auditivos. Si bien la rifaximina no está autorizada en estos casos, dado el reciente estudio aleatorizado controlado que mostró mejor evolución de la EH y menor mortalidad en pacientes que reciben lactulosa con rifaximina, los autores recomiendan su empleo preferencial. Los pacientes que se recuperan de un primer episodio de EHS generalmente necesitan tratamiento de mantenimiento con lactulosa.
Inducción y tratamiento de mantenimiento para la EHS recidivante (> 2 episodios)
El tratamiento de la EHS recidivante exige evaluar los factores desencadenantes. Además, se debe examinar la medicación de mantenimiento. El ajuste inapropiado de la dosis de lactulosa para lograr tres deposiciones diarias y la falta de cumplimiento son frecuentes. La educación del paciente es una parte importante del tratamiento. Si aparecen síntomas como distensión abdominal y diarrea excesiva, la lactulosa se debe reemplazar por rifaximina o neomicina.
Cuando aparece EHS mientras se recibe lactulosa en dosis adecuadas, se debe agregar rifaximina o neomicina, aunque se prefiere la rifaximina. En pacientes que sufren EHS episódica recidivante a pesar de dosis apropiadas de lactulosa y rifaximina, se debe buscar un gran shunt portosistémico intrabdominal mediante TC con contraste.
En el estudio de Sharma et al sobre prevención secundaria con lactulosa, la frecuencia de estos shunts espontáneos fue del 23% (32 de 140) pero se los puede hallar en hasta el 70% de los pacientes con EH persistente. En estos pacientes se debe tener en cuenta el riesgo de nefropatía debida a la TC con contraste. La angiografía por resonancia magnética es una alternativa para pacientes con filtración glomerular mayor de 30 ml/min por m2 de superficie corporal. Para pacientes bien compensados con cirrosis clase A de Child-Turcotte-Pugh o puntuaciones MELD bajas (<12-15), se debe considerar la embolización percutánea del shunt, que puede causar la remisión de la EHS recidivante en el 59- 90% de los pacientes. Si no se encuentra shunt o la puntuación MELD alta impide la embolización, serán necesarios otros tratamientos, tales como zinc, LOLA y alimentación con AACR.
Una vez que se logra la resolución de la EHS episódica, se debe mantener a todos los pacientes con EHS recidivante con lactulosa, rifaximina o ambas, según su tratamiento inicial.
Tratamiento de la EH grave (Grado 3 o 4 según los criterios de West Haven)
La EH grado 3 o 4 es una entidad grave que exige el monitoreo en una unidad de cuidados intensivos. Si el paciente tiene disminución de la conciencia serán necesarias la intubación endotraqueal y la respiración asistida. Tras la evaluación exhaustiva de los desencadenantes se debe administrar lactulosa por sonda nasogástrica (NG), 15 - 30 cc cada 1 a 2 horas hasta lograr 3 deposiciones.
Si no se dispone de acceso NG u orogástrico, se puede dar como enema (300 cc de lactulosa [10 g/15 ml] in 700 cc de agua esterilizada). Esto se puede repetir según necesidad, aunque se deben evitar las deposiciones demasiado flojas o voluminosas.
Junto con la lactulosa se administrará rifaximina. Los pacientes con diagnóstico de EHS, con tratamiento de los factores desencadenantes y que no responden a la lactulosa y los antibióticos no absorbibles deben ser revaluados a fin de asegurarse que el diagnóstico es correcto.
Si hay dudas diagnósticas, la TC craneal y el EEG pueden ser útiles para descartar hemorragia del sistema nervioso central o status epilepticus no convulsivo, respectivamente. Si el diagnóstico de EHS es correcto, se debe evaluar si el paciente tiene un gran shunt portosistémico y tratarlo.
La mayoría de estos pacientes no tienen un gran shunt y se los puede tratar con AACR, zinc y LOLA. Por último, para los pacientes que no responden a este enfoque se debe considerar el MARS. Los pacientes en recuperación de una EHS grave deben seguir un programa de mantenimiento con lactulosa y rifaximina.
Instrucciones sobre la conducción de vehículos para pacientes ambulatorios
La EHM es un factor de riesgo para accidentes al conducir vehículos. Es probable que una proporción considerable de pacientes hospitalizados por un episodio de EH y que se recuperan, padezcan encefalopatía mínima residual tras el alta. Por lo tanto, es importante aconsejar al alta sobre los riesgos de conducir vehículos. Bajaj et al evaluaron a 167 pacientes cirróticos prospectivamente durante un año e identificaron 18 accidentes a través de los archivos del Departamento de Transportes. De ellos, 16 (89%) se produjeron en pacientes que sufrían EHM según el Inhibitory Control Test y 8 de 18 (44%) en pacientes con EHM según las pruebas psicométricas estándar. A la inversa, es importante señalar que se diagnosticó EHM a aproximadamente el 55% de los pacientes que no tuvieron ningún accidente automovilístico.
Otro estudio evaluó la capacidad de conducir en la vida real en controles (n = 8), pacientes con cirrosis sin encefalopatía (n = 10), pacientes con EHM (n = 27) y pacientes con EHS grado 1 (n = 14). La capacidad para conducir fue del 87% en los controles, del 75% en los no EH, del 48% en los con EHM y del 39% en los que sufrían EH grado 1.Un estudio anterior halló considerable disminución de la capacidad, adaptación y cautela para manejar el automóvil en pacientes con EHM en relación con los controles o con pacientes cirróticos sin EHM. El instructor debió intervenir en 5 de 14 pacientes con EHM para evitar un accidente.
Se recomienda que los médicos tratantes efectúen las pruebas para EHM si se encuentran en centros de atención terciaria con acceso a estas pruebas. Los pacientes en quienes se comprueba EHM deben acudir a la agencia que entrega los registros de conducir para que se evalúe su capacidad.
Conclusión
En resumen, a la larga se produce EH en hasta el 50% de los pacientes con cirrosis. Los pacientes con EHS episódica en general son hospitalizados. Su tratamiento se puede dividir en dos fases: tratamiento de inducción y mantenimiento de la remisión. La lactulosa sigue siendo fundamental en ambas fases.
Actualmente hay evidencia que avala el empleo de rifaximina como tratamiento complementario de la EHS grave, pero aún no está autorizada por la FDA para esta indicación. La neomicina también se puede emplear como tratamiento complementario, pero es una opción menos atractiva debido a sus efectos secundarios adversos.
La embolización percutánea de grandes shunts portosistémicos y el tratamiento MARS son modalidades más nuevas, con evidencia que apoya su empleo para la EHS resistente al tratamiento médico. La mayoría de los pacientes necesitan tratamiento de mantenimiento tras el alta del hospital. Su educación y la de sus familiares sobre la importancia de estos medicamentos es esencial. Se debe agregar rifaximina al tratamiento con lactulosa para los pacientes con EHS recidivante. Es necesario asesorar a todos los pacientes y sus familias sobre los riesgos de accidentes al conducir vehículos. Es recomendable evaluar en estos pacientes su capacidad para conducir.
♦ Traducción y comentario objetivo: Dr. Ricardo Ferreira
