Introducción
El cáncer de mama es el cáncer más comúnmente diagnosticado en la mujer en los Estados Unidos y el segundo más letal [1]. Una de cada 8 mujeres será diagnosticada con cáncer de mama durante el transcurso de su vida. El manejo quirúrgico del cáncer de mama continúa evolucionando y sigue siendo un componente clave del tratamiento y la curación.
Esta revisión intenta brindar un panorama sobre el diagnóstico, tratamiento y seguimiento de las pacientes con cáncer de mama para el cirujano general.
Factores de riesgo
Muchos factores de riesgo para el desarrollo del cáncer de mama han sido identificados [2]. La herramienta más comúnmente usada para la evaluación del riesgo es la Breast Cancer Assessment Tool (modelo Gail), desarrollada por el National Cancer Institute y el National Surgical Adjuvant Breast and Bowel Project (NSABP) [3], que ha sido recientemente actualizada para estimar mejor el riesgo para las mujeres afroamericanas, de las islas del Pacífico e hispanas [4-6]. Incluye edad, raza, biopsia mamaria previa y resultados, edad de la menarca, edad al momento del primer parto vivo y parientes de primer grado con cáncer de mama. Esta herramienta puede ayudar como guía para la toma de decisión clínica y asistir para formular recomendaciones personalizadas (http://cancer.gov/bcrisktool/).
La quimioprevención con tamoxifeno o clorhidrato de raloxifeno debería ser considerada para todas las pacientes con carcinoma lobular in situ, hiperplasia ductal atípica, un modelo Gail de riesgo mayor que 1,7% o mutaciones BRCA1 (113705 OMIM) y BRCA2 (600185 OMIM).
El ensayo STAR (Study of Tamoxifen and Raloxifene) mostró que ambos, el tamoxifeno y el raloxifeno, reducen el riesgo de cáncer mamario invasor en un 50%, pero sólo el tamoxifeno ha probado que reduce el riesgo del desarrollo del carcinoma ductal in situ (CDIS) [7-9].
El carcinoma lobular in situ y la hiperplasia lobular atípica son conocidos colectivamente como neoplasia lobular [10]. La neoplasia lobular es un hallazgo histológico de atipia celular, que confiere un riesgo de 8 a 10 veces mayor para el desarrollo del cáncer mamario invasor, en cada mama [11]. Las mujeres con carcinoma lobular in situ deberían ser consideradas para el tratamiento con tamoxifeno, que ha demostrado reducir el riesgo en un 56% [12].
La hiperplasia ductal atípica es una lesión premaligna que confiere un riesgo de 4 a 5 veces mayor para el cáncer de mama. La quimioprevención con tamoxifeno es efectiva, reduciendo el riesgo en un 86%.
Las mutaciones genéticas conocidas se encuentran en el 5% de las mujeres con cáncer mamario familiar e incluyen los genes de alta penetración TP53 (síndrome de Li-Fraumeni) (191170 OMIM), PTEN (síndrome de Cowden) (601728 OMIM) y las mutaciones BRCA, así como muchos genes de baja penetración [13]. De esos, las mutaciones genéticas del BRCA son las más prominentes. Otro 10% de los cánceres son familiares, sin mutación genética conocida [14].
La National Comprehensive Cancer Network ha publicado guías para la remisión de pruebas adicionales y asesoramiento genético. Los criterios en una paciente sin un diagnóstico conocido de cáncer de mama, incluyen 2 o más cánceres primarios de mama sobre el mismo lado de la familia (en el mismo individuo o en 2 individuos separados), 1 o más cánceres de ovario en el mismo lado de la familia, cáncer de mama en una pariente de primero o segundo grado diagnosticado antes de los 45 años de edad, mutación conocida en un gen susceptible para el cáncer de mama en la familia y cáncer de mama masculino [15].
Los detalles completos de las guías pueden hallarse en la página en internet de la National Comprehensive Cancer Network [15]. Se recomienda a las pacientes con un riesgo familiar alto, un asesoramiento genético y potencialmente pruebas genéticas, vigilancia aumentada y posiblemente quimioprevención. Ellas también podían presentarse con las opciones de mastectomía profiláctica bilateral y ooforectomía, lo que se ha asociado con una mejor sobrevida específica para el cáncer de mama y sobrevida global [14,16].
Los genes BRCA fueron descritos por primera vez en 1994 [17,18]. Su descubrimiento ha brindado un significativo entendimiento de la biología del cáncer de mama. Ambas mutaciones, BRCA1 y BRCA2, confieren un 60% a 80% de riesgo durante el curso de vida para el desarrollo de cáncer de mama y se asocian con otras neoplasias malignas, incluyendo el cáncer de ovario.
El BRCA1 está ligeramente más fuertemente asociado con el cáncer de ovario que el BRCA2, mientras el BRCA2 está más altamente asociado con el cáncer pancreático y el cáncer de mama en el hombre [19]. Por lo menos, una mujer con mutaciones BRCA conocidas debería someterse a una vigilancia aumentada que incluya mamografía y resonancia magnética bilateral de mamas anuales [15]. Además de la mayor vigilancia, el tamoxifeno puede brindar una reducción del riesgo en pacientes con el gen BRCA, aunque eso no ha sido nunca específicamente abordado en los ensayos clínicos.
Alternativamente, las pacientes con esas mutaciones pueden ser consideradas para la mastectomía bilateral y ooforectomia bilateral [20].
Detección y Diagnóstico
A pesar de ensayos randomizados mostrando una mortalidad descendida con la mamografía de detección [21-23], ha existido una considerable controversia alrededor de las guías de detección en los Estados Unidos, en los últimos años.
Las recomendaciones más recientes de la American Cancer Society abogan por la mamografía anual de detección, comenzando después de los 40 años [24]. Las guías de la US Preventive Services Task Force, publicadas en 2009, recomiendan mamografía de detección bianual para las mujeres de 50 a 74 años, desalentando los auto-exámenes mamarios y concluyendo que la evidencia es insuficiente para recomendar un examen clínico mamario además de la mamografía, en mujeres de 40 a 50 años [25,26]. Esto ha causado considerable confusión entre las pacientes y debate entre los profesionales [27]. No obstante, muchos grupos (American College of Surgeons, American College of Radiology, etc.) avalan las guías de detección recomendadas por la American Cancer Society.
La mamografía de detección está lejos de ser una prueba perfecta, con una sensibilidad que puede ser tan baja como del 70% en una mujer menor de 50 años [28,29]. La evidencia sobre los beneficios de la mamografía de detección en mujeres mayores de 50 años es clara, con una reducción de la mortalidad de hasta un tercio [30]. Los falsos positivos son un problema significativo y son estimados en hasta el 30% de las poblaciones examinadas, llevando a costosos procedimientos invasivos y estrés psicológico para la paciente [31]. Sin embargo, por cada 2.000 mujeres examinadas, 1 tendrá prolongada su vida y 10 serán sometidas a procedimientos innecesarios [32].
La mayoría de los profesionales continúan siguiendo las guías de la American Cancer Society y la tasa de mamografía no ha tenido un cambio significativo desde la publicación de las guías de la US Preventive Services Task Force [33]. Independientemente de la guía usada, cualquier régimen de detección requiere educación del paciente sobre los riesgos y beneficios [29,34].
Mamografía digital y resonancia magnética
La subutilización de los programas de detección mamográfica es una preocupación en curso de la salud pública [35]. Los programas de detección han demostrado ser efectivos para reducir la mortalidad entre las pacientes de bajos ingresos, sin seguro médico o con baja cobertura [36].
Las guías para el uso de la mamografía digital y de la resonancia magnética, han evolucionado dramáticamente durante la década pasada. Tanto las guías de la American Cancer Society, como las de la US Preventive Services Task Force, incluyen esas modalidades en sus recomendaciones, aunque con diferentes interpretaciones sobre su utilidad.
La mamografía digital es más certera que la mamografía convencional en mujeres menores de 50 años, con mamas densas y premenopáusicas [28,37].
La resonancia magnética es recomendada, en la actualidad, como una modalidad de detección en mujeres jóvenes que tienen un riesgo alto de desarrollar cáncer de mama, definido por sus antecedentes familiares u otros factores que, cuando se combinan, confieren un riesgo de por vida de más del 20% [38,39].
Es útil para evaluar la respuesta al tratamiento neoadyuvante, pero raramente reduce el número de operaciones o cambios en el plan de tratamiento quirúrgico y su certeza varía con el fenotipo molecular y tipo de tratamiento neoadyuvante [40-42].
Otras modalidades emergentes de imágenes están actualmente bajo investigación. Se considera que la tomosíntesis mamaria es equivalente a la mamografía estándar y la ecografía automatizada de toda la mama es confiable para la detección de lesiones mayores de 1,2 cm. Estudios adicionales a escala mayor están garantizados [43,44].
Una vez que una paciente tiene una anomalía sospechosa en la mamografía de detección, el primer paso debería ser una mamografía diagnóstica con o sin ecografía; si se la detecta en una resonancia magnética, el paso siguiente es la ecografía. Las anomalías confirmadas con la mamografía diagnóstica o la resonancia magnética, deberían ser evaluadas con una biopsia con aguja gruesa guiada por imágenes.
Biopsia, histología
La hiperplasia lobular atípica se asocia con un cáncer subyacente en el 13% de los especímenes [45] y cuando la hiperplasia ductal atípica es hallada en la biopsia con aguja gruesa, la paciente debería ser sometida a una biopsia por resección, dado que hasta el 30% de las pacientes tendrán un CDIS o un cáncer invasor, en la biopsia por resección [46-48].
Varios otros hallazgos histológicos en la biopsia con aguja gruesa pueden estar asociados con una neoplasia maligna. Los mismos incluyen la atipia epitelial plana (que usualmente es hallada en asociación con hiperplasia ductal atípica o CDIS), lesiones papilares y cicatriz radial. Esos hallazgos deberían ser discutidos con un equipo multidisciplinario y formularse un plan de tratamiento específico para la paciente [46,49]. En general, si la atipia o la cicatriz radial son halladas en la biopsia con aguja gruesa, la biopsia por resección debería ser considerada.
El tejido es enviado para examen histopatológico con receptores hormonales y tinción HER-2/neu si se encuentra cáncer. El estatus del receptor de estrógeno (RE) y del receptor de progesterona y del HER-2/neu afecta tanto al pronóstico como a las opciones terapéuticas [50].
El carcinoma ductal invasor es, por mucho, el diagnóstico anatomopatológico más común, representando el 80% de todos los cánceres de mama.
El carcinoma lobular invasor, el segundo más común, representa el 10% de los cánceres mamarios. El cáncer inflamatorio de la mama es un subtipo agresivo, que se presenta con edema y eritema de la mama. Dado que representa la diseminación del cáncer en los linfáticos dérmicos, puede ser diagnosticado con biopsia de la piel con punch, pero es primariamente un diagnóstico clínico basado en la rápida progresión de los cambios en la piel. Casi todas las mujeres con ese subtipo de cáncer tienen compromiso ganglionar al momento del diagnóstico y un tercio tiene metástasis a distancia [51].
El CDIS es un cáncer que no ha violado la membrana basal, en otras palabras, es un cáncer preinvasor. La incidencia de CDIS ha escalado con la detección aumentada y la mejora en las modalidades de imágenes, aunque eso por sí solo no explica enteramente el aumento. Se lo ve más frecuentemente como calcificaciones en la mamografía [52]. El Van Nuys Prognostic Indicator puede ser usado para predecir el riesgo de recidiva local [53]. Los indicadores de mal pronóstico para la recidiva local incluyen: grado alto, comedonecrosis, márgenes positivos y edad joven.
Otras variantes histológicas (medular, tubular, papilar y mucinoso) generalmente son tratadas de manera similar al carcinoma ductal invasor, aunque frecuentemente tienen un mejor pronóstico [54,55]. Otros dos tipos raros pero importantes de cánceres de mama, son la enfermedad de Paget y el tumor filoides [42].
La enfermedad de Paget se manifiesta con un pezón enrojecido, escamoso y urticante y se diagnostica con una biopsia del área afectada. Los estudios más recientes muestran que entre el 85% y el 92% de las mujeres con enfermedad de Paget tienen un cáncer de mama subyacente [56,57]. Cada diagnóstico de enfermedad de Paget debería ser seguido por una búsqueda del cáncer.
El tumor filoides (también llamado cistosarcoma filoides) es una neoplasia rara de la mama, un tumor fibroepitelial que se origina del estroma periductal [58]. Los tumores filoides son calificados como benignos, limítrofes o malignos. Tienden a recidivar localmente, por lo que aún los tumores filoides benignos deben ser resecados con un amplio margen (≥1 cm) [58].
Por décadas, el cáncer invasor de mama se ha caracterizado por grados (1, 2 o 3) y por si las células del tumor tienen receptores para estrógeno y/o progesterona. La expresión de la proteína HER-2/neu también es rutinariamente señalada, ya que cada una de esas características es usada para determinar la necesidad y el tipo de terapia sistémica. Los cánceres de mama más invasores tienen receptores para estrógeno y progesterona (cánceres hormono-sensibles).
El subtipo molecular es ampliamente usado clínicamente en la actualidad para el cáncer de mama invasor. El Oncotype DX (Genomic Health) es un estudio de 21 genes para RE positivo, ganglio linfático negativo, tejido fijado con formol, que predice el riesgo de recidiva y la respuesta al tamoxifeno [59-61).
Recientemente, ha demostrado ser útil también en pacientes con ganglios linfáticos positivos. Esta prueba puede preservar a algunas pacientes de la quimioterapia [62]. El MammaPrint (Agendia) es un estudio de 70 genes para RE positivo y negativo, ganglio linfático negativo, tejido fijado con formol y embebido en parafina, que predice el riesgo de recidiva y es usado para estratificar pacientes que podrían beneficiarse con la quimioterapia adyuvante [63].
El PAM 50 Breast Cancer Intrinsic Classifier (Laboratorios ARUP) es un estudio de 50 genes para cáncer en estadio temprano, ganglio linfático negativo, tejidos fijados con formol, que clasifica el cáncer de mama en 1 de 5 subtipos moleculares aceptados [64,65]. Estos perfiles pueden ser usados para predecir el riesgo de recidiva y la respuesta a la quimioterapia adyuvante o neoadyuvante, así como para brindar una información pronóstica general [65-67].
Tratamiento del cáncer de mama
El tratamiento del cáncer de mama es multidisciplinario y puede incluir cirugía, radiación y terapia sistémica (quimioterapia, terapia hormonal o terapia biológica). Varios factores deberían ser usados para determinar no sólo los tratamientos a considerarse, sino también la secuencia de las terapias.
En la mayoría de las pacientes con cáncer de mama en estadio temprano (CDIS y tumores pequeños que tienen clínicamente ganglios linfáticos negativos) se procederá primero con la cirugía. En pacientes con tumores grandes, compromiso de la piel o enfermedad ganglionar voluminosa, muchos aconsejan quimioterapia neoadyuvante, dado que la respuesta a la quimioterapia brinda información pronóstica.
Las pacientes con cáncer inflamatorio de mama deberían recibir siempre primero quimioterapia. La radiación se brinda después de la quimioterapia y cirugía, excepto en pacientes sometidas a irradiación parcial de la mama, cuando se la utiliza después de la cirugía pero antes de la quimioterapia. Para pacientes más añosas o enfermas con tumores hormono-positivos, la terapia endócrina puede ser usada como único tratamiento.
El CDIS requiere una mención especial. El manejo de la mama con CDIS es similar al del cáncer invasor en estadio temprano, aunque las pacientes con riesgo bajo pueden someterse sólo a la tumorectomía.
Aproximadamente el 15% de las pacientes con CDIS tendrá un cáncer invasor asociado [68]. Como fuera mencionado previamente, para las pacientes CDIS con RE positivo, el tamoxifeno es útil para disminuir el riesgo de cáncer invasor ipsilateral.
Manejo quirúrgico de la mama
La cirugía ha sido la modalidad primaria para el tratamiento del cáncer de mama por siglos. Halsted, que fue el primero en realizar una mastectomía radical en 1892, publicó sus resultados en 1894 [69]. El abordaje Halstediano permaneció como el gold standard para el manejo quirúrgico del cáncer de mama por cerca de 80 años.
En los últimos 30 años, el manejo quirúrgico de la mama ha hecho una transición desde los abordajes radicales a procedimientos menos extremos, conservadores de la mama, aunque recientemente ha habido una tendencia al aumento de la tasa de mastectomía, por razones no claras [70]. La evolución hacia procedimientos menos radicales se ha basado en evidencia.
Un ensayo randomizado, el NSABP B-04, que comenzó en 1971, comparó la mastectomía radical sin radiación vs la mastectomía simple con radiación vs la mastectomía simple sin radiación. Enroló 1.765 pacientes en 126 meses y el esquema randomizado se basó parcialmente en el estado ganglionar, con pacientes con enfermedad ganglionar linfática “clínicamente” positiva randomizadas para mastectomía radical o mastectomía simple con radiación. Las pacientes con ganglios linfáticos “clínicamente” negativos fueron randomizadas para cualquiera de los tres grupos. No se vieron diferencias entre los grupos en sobrevida, libre de enfermedad, o libre de enfermedad distante y ese estudio llevó al final de la era de la mastectomía radical [71].
Otro ensayo, el NSABP B-06, involucró la comparación randomizada de la mastectomía vs tumorectomía con radiación, para las pacientes que tenían enfermedad en estadio I o II, con tumores menores de 4 cm. En total, 2.163 mujeres fueron enroladas desde 1976 hasta 1984 y la disección axilar se efectuó en todos los grupos. El seguimiento alejado a 20 años de 1.851 pacientes fue publicado en 2002. No hubo diferencias en la sobrevida global, pero hubo diferencias significativas en la recidiva, teniendo el grupo de sólo tumorectomía una tasa de 39,2% de recidiva a 20 años [72].
Por lo tanto, la tumorectomía con radiación se ha convertido en el estándar para la cirugía conservadora de la mama. Las contraindicaciones absolutas para la tumorectomía incluyen radioterapia previa, embarazo, tumor multicéntrico, imposibilidad para obtener márgenes claros (tumores grandes o invasión dentro de estructuras adyacentes) e inhabilidad física para tolerar la radioterapia.
Las contraindicaciones relativas incluyen tumor multifocal, enfermedad del tejido conectivo y un tumor grande para el tamaño de la mama. Las recidivas deberían ser manejadas con mastectomía.
La mastectomía con preservación de piel ha demostrado ser oncológicamente segura en pacientes seleccionadas [75]. La mastectomía con conservación del pezón es más controversial, pero está ganando aceptación [74]. La mayoría de los estudios actuales apoyan su seguridad con un seguimiento de hasta 5 años, pero el seguimiento a largo plazo aún no está disponible [75-77].
El uso de la mastectomía profiláctica contralateral ha aumentado en los últimos años. Las razones para ello son multifactoriales, pero se originan en parte en el malentendido de las pacientes sobre el riesgo global de desarrollar un cáncer contralateral [78,79].
La mastectomía contralateral profiláctica está más justificada en pacientes con un riesgo alto de por vida para desarrollar cáncer contralateral de mama (BRCA positivo, fuertes antecedentes familiares o paciente joven con enfermedad biológicamente agresiva).
Manejo quirúrgico de la axila
En cualquier discusión del manejo quirúrgico de la axila, es importante señalar que la remoción de los ganglios linfáticos no trasmite beneficio en el tratamiento de la paciente y sólo es para estadificación y pronóstico. En el tratamiento de la mama, el manejo de la axila ha tendido a hacer menos con el paso del tiempo.
El trabajo innovador de Morton y col. [80], en la biopsia del ganglio linfático centinela en el melanoma, pavimentó el camino para la aplicación de esa técnica a otras neoplasias malignas. Su uso en el cáncer de mama fue establecido por Guiliano y col. [81,82], y en el histórico estudio NSABP B-32 [83].
El estudio NSABP B-32 comparó la biopsia del ganglio linfático centinela seguida por disección axilar, con la biopsia del ganglio centinela seguida por disección axilar sólo si el ganglio linfático centinela era positivo. Ese avance revolucionó el tratamiento quirúrgico de la axila y por más de una década, el estándar siguió siendo la biopsia del ganglio centinela en la axila clínicamente negativa, seguida por disección de los ganglios linfáticos axilares, en aquellas con ganglio centinela positivo. No obstante, se conoció, del NSABP B-32 y otros estudios, que un gran número de mujeres con ganglio linfático centinela positivo, podían no tener otros ganglios linfáticos positivos en la disección completa.
Los nomogramas para predecir la probabilidad de ganglios linfáticos adicionales (no centinela) positivos, fueron desarrollados y usados ampliamente en la clínica [85,85]. Con el uso cada vez mayor de las terapias adyuvantes, las pacientes sometidas sólo a la remoción de los ganglios linfáticos centinelas, sin completar con la disección axilar, no tuvieron tasas altas de recidiva regional, aunque eso no ha sido probado de una manera randomizada [86].
El ensayo ZO011 del American College of Surgeons Oncology Group, mostró que – en pacientes seleccionadas – la disección axilar para completar, podía ser omitida con seguridad [88.89]. Las pacientes con cáncer de mama en estadio I o II sometidas a terapia conservadora de la mama, con menos de 3 ganglios linfáticos positivos, no tienen diferencias en la sobrevida y recidiva en un seguimiento promedio de 6,3 años.
Ese estudio condujo a un cambio generalizado en el manejo de la axila en las pacientes que entraban en el criterio de selección [90,91]. Para reiterar, en pacientes con tumores de menos de 5 cm, con axila clínicamente negativa, menos de 4 ganglios positivos y tratamiento con terapia conservadora de la mama (no incluye la radiación de toda la mama) y terapia hormonal sistémica adyuvante o quimioterapia, la completitud de la disección de los ganglios linfáticos axilares puede ser omitida con seguridad.
La biopsia del ganglio linfático centinela está indicada en cualquier cáncer invasor con axila clínicamente negativa. Los estudios también apoyan la biopsia del ganglio linfático centinela en pacientes sometidas a mastectomía por CDIS o lesiones de alto riesgo [92]. El ensayo NSABP B-27 y otros estudios mostraron que la biopsia del ganglio linfático centinela puede ser certera después de la terapia neoadyuvante [93,94].
Estadificación
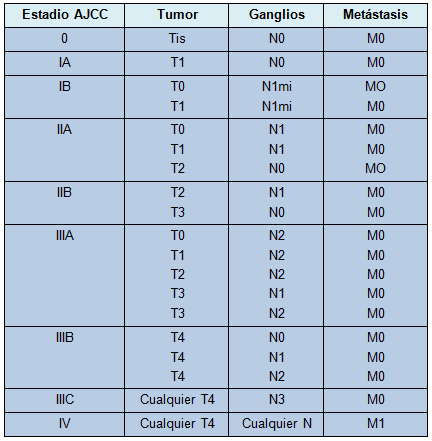
La cirugía sigue siendo el método más seguro para estadificar los tumores malignos no metastásicos. El sistema de estadificación para el cáncer de mama sigue al sistema TNM. El sistema más recientemente aprobado por el American Joint Committee on Cancer se muestra en la Tabla 1 [95]. El pronóstico basado en el tamaño y estado ganglionar, sin una comprensión de la biología individual del tumor subyacente, es imperfecto.
• TABLA 1: Estadificación simplificada del cáncer de mama del AJCC adaptada de la 7º edición
Quimioterapia
En la era de los perfiles moleculares, la decisión de ofrecer quimioterapia está basada cada vez más en el análisis del tumor del individuo [67]. Aparte del perfil molecular, las indicaciones relativas para la quimioterapia incluyen tamaño tumoral grande (>2 cm), ganglios linfáticos positivos, tumores RE negativo y receptor de progesterona negativo, tumores HER-2/neu positivos y cáncer inflamatorio de la mama [60,67].
Los regímenes basados en antraciclina han demostrado ser superiores a los basados en metotrexato, aunque los efectos adversos son más sustanciales [96-98]. La adición de taxanos a la quimioterapia ha demostrado mejorar significativamente los resultados y es usada en la actualidad para el tratamiento estándar [102-104].
Las indicaciones para la terapia neoadyuvante se han expandido mucho en los pasados 10 años, con guías de consenso internacional publicadas [105]. Un ensayo, el NSABP B-27, mostró que las pacientes con un respuesta patológica completa a la quimioterapia neoadyuvante, tenían una sobrevida global y libre de enfermedad mejoradas [106].
Radioterapia
La radiación después de la tumorectomía se asocia con una tasa baja de recidiva [72]. En pacientes que han tenido una mastectomía, el tamaño grande del tumor (>5 cm), 4 o más ganglios linfáticos positivos, márgenes estrechos o cáncer inflamatorio, son todas indicaciones para la radiación adyuvante [107]. A las mujeres con cáncer recidivado que no habían tenido una radiación previa, se les debería también ofrecer esa terapia. La radiación consiste típicamente en la irradiación de toda la mama por 6 a 6 ½ semanas, incluyendo una dosis de refuerzo.
La radiación acelerada parcial de la mama ha sido recientemente introducida y la American Society for Radiation Oncology ha publicado guías para el uso de esa tecnología [108]. Mientras existen múltiples sistemas de entrega para esta terapia, no todos han sido evaluados en ensayos clínicos. El dispositivo MammoSite (Hologic), que involucra la colocación de un catéter balón en el sitio de la tumorectomía, ha sido evaluado en el American Society of Breast Surgeons MammoSite Breast Brachyterapy Registry Trial [109].
El dispositivo Intrabeam (Carl Zeiss), usado para entregar radiación al lecho del tumor intraoperatoriamente, ha sido evaluado en el Targeted Intraoperative Radiotherapy for Breast Cancer Trial (TARGIT) [110]. Cuando se compara con la irradiación de toda la mama, existe una tasa similar de recidiva local con ambos dispositivos. Quedan por resolverse las preocupaciones sobre la formación aumentada de seromas y problemas en la herida con esas tecnologías [111-113].
El hipofraccionamiento, en donde dosis grandes de radiación son brindadas en unos pocos tratamientos, es también un área de gran interés que necesita estudio adicional. Múltiples estudios en curso deberían resolver algunas de las cuestiones remanentes, relacionadas con el uso apropiado de la irradiación acelerada parcial de la mama e hipofraccionamiento.
Bloqueo hormonal y terapia biológica
El RE fue identificado por primera vez a fines de la década de 1950 y las terapias endócrinas han sido usadas en el tratamiento de los cánceres de mama por más de 60 años. Todas las pacientes con tumores RE positivos deberían ser tratadas con adyuvante bloqueador RE. La positividad del receptor de progesterona, aún en tumores RE negativos, es un fuerte predictor de respuesta al tamoxifeno [50].
La droga más ampliamente usada y estudiada en esa categoría es el tamoxifeno. La recomendación actual es para 5 años de tratamiento, aunque un curso más largo, de 10 años, ha demostrado una reducción adicional del riesgo de recidiva y mortalidad [114].
El tamoxifeno fue comparado con el inhibidor de la aromatasa, el anastrozol, en el ensayo ATAC (Arimidex, Tamoxifen, Alone or in Combination) [115]. Ese estudio mostró que el anastrozol era superior (sobrevida libre de enfermedad prolongada, aumento del tiempo de recidiva y disminución de las metástasis a distancia y de los cánceres mamarios contralaterales), con pocos efectos adversos, comparado con el tamoxifeno, cuando se usó en mujeres postmenopáusicas con cánceres con respuesta estrogénica.
Por lo tanto, los inhibidores de la aromatasa son los agentes de bloqueo hormonal de elección en las mujeres postmenopáusicas. Los inhibidores de la aromatasa no deberían ser usados en mujeres premenopáusicas, en las que el estrógeno es producido principalmente por los ovarios, dado que activa el eje hipotálamo-hipofisario, con producción aumentada de andrógenos ováricos, que contrarrestan los efectos de la droga.
Las terapias biológicas específicas son, tal vez, usadas más ampliamente en el cáncer de mama que en cualquier otra enfermedad. La amplificación del receptor HER-2/neu en el cáncer de mama fue reconocida primero en las líneas celulares en el cáncer mamario. Luego fue evaluada en cánceres de mama humanos y su presencia confirió un mal pronóstico [116]. Se asocia con tasas más altas de recidiva, resistencia relativa a la terapia hormonal y resistencia a algunos quimioterápicos [117].
Todos los reportes anatomopatológicos deberían incluir el estatus HER-2/neu por inmunohistoquímica, con resultados equívocos (por ej., 2+) confirmados por análisis de hibridación fluorescente in situ. El desarrollo de un anticuerpo monoclonal contra el HER-2/neu, el trastuzumab, revolucionó el tratamiento para el 15% al 20% de las pacientes con cánceres de mama HER-2/neu positivos.
Múltiples estudios han probado la eficacia de esa droga en la dramática mejora de los resultados, en pacientes con cánceres HER-2/neu positivos [118-122]. El uso del trastuzumab es recomendado en pacientes con amplificación confirmada del gen HER-2/neu (3+ en la inmunohistoquímica o confirmado por análisis de hibridación fluorescente in situ). Las pacientes con tumores HER-2/neu positivos son tratadas con trastuzumab por 1 año.
Se han desarrollado drogas adicionales específicas para el HER-2/neu. El pertuzumab inhibe la dimerización del receptor HER-2/neu y es complementario del trastuzumab cuando se usan en combinación [123.124]. El lapatinib es específico para el receptor del factor de crecimiento y HER-2/neu, pero tiene resultados inferiores al trastuzumab cuando se lo utiliza solo [125]. El trastuzumab se asocia con riesgo de insuficiencia cardíaca y las pacientes deben someterse a evaluación de la función cardíaca antes de comenzar la terapia [126,127].
El uso de inhibidores del factor de crecimiento del endotelio vascular, tales como el bevacizumab, para el tratamiento del cáncer de mama sigue siendo controversial [128]. Una discusión de esta cuestión va más allá del alcance de esta revisión.
Seguimiento
La American Society of Clinical Oncology recomienda que las pacientes sean vistas en la clínica cada 3 a 6 meses durante los primeros 3 años, cada 6 a 12 meses en el 4º y 5º año y luego anualmente. Las pacientes que han sido sometidas a cirugía conservadora de la mama con radiación, deberían tener su primera mamografía post tratamiento 1 año después de la mamografía que llevó al diagnóstico, pero no antes de los 6 meses después de completar la radioterapia. Luego, las mamografías deberían ser realizadas anualmente [129].
Conclusión
El cáncer de mama es una enfermedad común pero extremadamente compleja. Deben hacerse cuidadosas consideraciones sobre la naturaleza única de cada tumor y paciente. Cada cáncer de mama recién diagnosticado debería ser presentado en una conferencia multidisciplinaria, para asegurar el manejo óptimo por todas las especialidades involucradas. El tratamiento del cáncer de mama continuará evolucionando; las mejoras adicionales en los resultados se basarán probablemente en el desarrollo de terapias específicas.
♦ Comentario y resumen objetivo: Dr. Rodolfo D. Altrudi