|
Puntos Claves • Las opciones para la manipulación menstrual son regímenes extendidos o continuos de anticonceptivos hormonales orales, transdérmicos o vaginales; un dispositivo intrauterino que libera levonorgestrel; el implante de progestina, y las inyecciones de medroxiprogesterona de hormonales. • Los beneficios incluyen la menos síndromes menstruales relacionados, menor ausentismo laboral o escolar y, en general, mayor satisfacción. Las indicaciones médicas son las condiciones exacerbada por los cambios hormonales en la época de las menstruaciones. • La desventaja principal es la tasa más elevada de sangrado menstrual. • Los mitos y las percepciones erróneas acerca de la manipulación menstrual siguen existiendo. |
Si así lo desean, las mujeres pueden tener más control sobre la frecuencia de las menstruaciones y su posible supresión. Mediante los regímenes con anticonceptivos hormonales extendidos o continuos pueden tener sus períodos con menos frecuencia, una práctica llamada manipulación o supresión menstrual.
En realidad, con la ayuda de sus médicos, las mujeres han estado haciendo esto durante años. En la actualidad existen varios productos aprobados en EE.UU por la Food and Drug Administration (FDA) específicamente para el uso prolongado o continuo. Esta práctica ha tenido una amplia aceptación.
Las razones para suprimir el flujo menstrual van desde evitar el sangrado durante un determinado evento (por ej., una boda, una graduación o deportes competitivos) a la búsqueda de alivio de la dismenorrea o la reducción o eliminación de la menstruación para el tratamiento de la endometriosis, la migraña, y otras condiciones médicas agravadas por los cambios hormonales en la época menstrual. Por otra parte, algunas mujeres pueden manipular sus menstruaciones sin ninguna razón, simplemente para evitar la menstruación.
• Los Trastornos Menstruales son Molestos y Comunes
En Estados Unidos, todos los años casi 2,5 millones de mujeres de 18 a 50 años se ven afectadas por trastornos menstruales como la dismenorrea (menstruación dolorosa), la menorragia (menstruación excesiva o frecuente), la metrorragia (menstruación irregular), la menometrorragia (menstruación excesiva e irregular) y el síndrome menstrual. En Estados Unidos, los trastornos menstruales son la causa principal de morbilidad ginecológica, triplicando el número de tumores anexiales (la segunda causa más importante). Por otra parte, estos trastornos afectan la vida laboral provocando grandes costos.
• Breve Historia de los Anticonceptivos
La idea de utilizar gestágenos para el control de la natalidad apareció en la década de 1.950, cuando Gregory Pincus propuso un régimen de 21 días de fármaco activo seguido de 7 días libre de fármacos, con el fin de provocar una hemorragia por deprivación, imitando al ciclo menstrual natural. Este régimen “21/7” fue diseñado siguiendo el ciclo lunar, con la esperanza de que podría ser, en palabras del Dr. John Rock “una variante moralmente admisible del método rítmico," haciéndolo aceptable para las mujeres, los clínicos y la Iglesia Católica.
En 1.977, Loudon et al. informaron los resultados de un estudio en el que las mujeres tomaron píldoras activas durante 84 días en lugar de 21 días, lo que redujo la frecuencia de la menstruación cada 3 meses. Desde entonces, la administración de pastillas activas durante más de 21días con el fin de evitar la menstruación y otros síntomas por supresión hormonal se ha convertido en una práctica clínica popular y son muchos los estudios que han investigado el uso prolongado o continuo de los anticonceptivos orales para retrasar las menstruaciones.
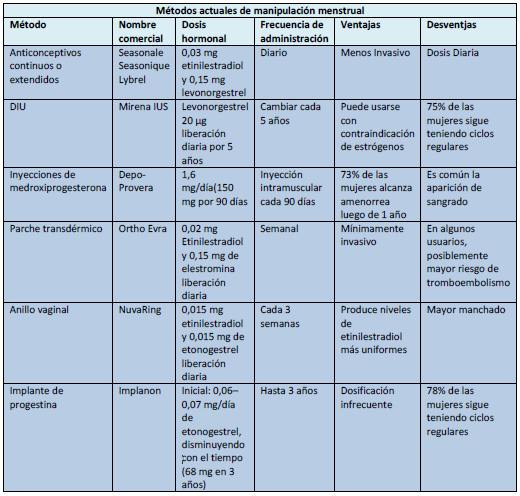
• Métodos Actuales de Manipulación de Menstrual
Existe una variedad de productos para prevenir la concepción al alterar el ciclo menstrual:
• Anticonceptivas orales de estrógeno y progestina
• Dispositivo intrauterino (DIU) de liberación prolongada
• Inyecciones de acetato de medroxiprogesterona de depósito
• Parche anticonceptivo transdérmico
• Anillo vaginal anticonceptivo
• Anticonceptivo implantable con etonogestrel.
Anticonceptivos orales
La manera más común de manipular el ciclo menstrual es prolongar el tiempo entre las semanas libres de hormonas mediante el uso de anticonceptivos orales.
Si la paciente es joven se puede prescribir un anticonceptivo oral monofásico 21/7 para ser tomado todos los días durante 21 días, seguido luego de un nuevo envase y así sucesivamente durante un máximo de 84 días consecutivos, saltando las píldoras placebo hasta que la mujer desee tener su período menstrual, quien también puede elegir la semana en que quiere tenerlo: si le parece que un régimen programado de anticonceptivos orales de 12 semanas es inconveniente, puede planificar la menstruación para las semanas 10, 9 o cualquier otra.
El justificativo para realizar un ciclo de 84 días (12 semanas) es que proporciona 4 períodos anuales, aliviando los temores de la hipertrofia del endometrio.
En este contexto, una hemorragia no programada o en curso puede controlarse tomando una pastilla "doble", de un envase de repuesto en cualquier día del sangrado en curso y hasta que se resuelva. No se deben programar períodos menstruales con intervalos más cortos de 21 días, debido al riesgo de ovulación. Los días que no se tomaron los comprimidos o se utilizaron píldoras de placebo también deben ser superiores a los 7 días para evitar un escape ovulatorio.
En algunas mujeres con endometriosis y otras razones médicas se pueden rescribir anticonceptivos orales continuos sin la semana deplacebo.
Lamentablemente, el inconveniente de suprimir la hemorragia por deprivación es un sangrado no programado. Se desconoce cuál es la mejor manera de tratar este sangrado no programado. Las pacientes que no son sexualmente activas pueden estar seguras de que todavía se puede lograr el objetivo de tener un endometrio atrófico por la amenorrea resultante de la píldora (especialmente útil para aquellas personas con dismenorrea severa u otras razones para querer evitar el flujo).
Las pacientes también podrían tratar de manejar el flujo suspendiendo periódicamente de 3 a 5 días las pastillas con hormonas, con el fin de permitir el flujo. También pueden tratar de cambiar por otro anticonceptivo oral que tenga una progestina diferente, con el potencial de espiralar con más fuerza las arteriolas del endometrio y por lo tanto inducir una atrofia más intensa. El levonorgestrel es 10 a 20 veces más potente que la noretindrona. La elección de una tableta con una dosis más elevada de levonorgestrel o una progestina monofásica similar puede minimizar un sangrado no programado.
En la actualidad, son varios los anticonceptivos orales aprobados para ser usados en regímenes prolongados.
Seasonable fue el primer anticonceptivo oral comercializado en Estados Unidos para un régimen activo extendido. Viene en envases con 84 comprimidos conteniendo 0,03 mg de etinilestradiol y 0,15 mg de levonorgestrel, más 7 pastillas de placebo.
Seasonique es similar al Seasonale, pero en lugar de 7 píldoras de placebo tiene 7 comprimidos con 0,010 mg de etinilestradiol.
Lybrel es una combinación en dosis bajas de 0,02 mg de de etinilestradiol y 0,09 mg de levonorgestrel. Se comercializa en envases para 1 año, el cual contiene pastillas activas para ser tomadas en forma continua durante 365 días, sin una fase de placebo o un intervalo libre de pastillas; es el único anticonceptivo continuo oral aprobado por la FDA y disponible en Estados Unidos.
Dispositivo intrauterino
Los dispositivos intrauterinos (DIU) fueron desarrollados originalmente como anticonceptivos. El agregado de una progestina ha demostrado que reduce el sangrado menstrual abundante hasta en un 90%.
Mirena IUS, un dispositivo liberador de levonorgestrel, es el único DIU disponible actualmente en Estados Unidos ("IUS" significa "sistema intrauterino"). Fue aprobado recientemente por la FDA para tratar el sangrado menstrual abundante en las mujeres que utilizan un método anticonceptivo intrauterino para evitar el embarazo. Alrededor del 50% de las mujeres que usan este dispositivo desarrollan amenorrea en un plazo de 6 meses a partir de la inserción, mientras que el 25% presenta oligomenorrea.
En la mayoría de las mujeres, el dispositivo Mirena puede dejarse en el útero durante 5 años. Puede ser una buena opción para inducir amenorrea en mujeres con trastornos de la hemostasia o en quienes están contraindicados los estrógenos o causan problemas de salud. El DIU de cobre sigue siendo una opción viable para las mujeres que no toleran el tratamiento hormonal. Sin embargo, Mirena puede provocar menos sangrado no programado que el DIU de cobre.
Inyección de acetato de medroxiprogesterona de depósito
Depo-Provera (acetato de medroxiprogesterona de depósito) se administra a intervalos de 90 días. Este método anticonceptivo inhibe la ovulación y libera al endometrio de deciduas, reduciendo o eliminando el sangrado uterino.
Si bien inicialmente los nuevos usuarios pueden experimentar el sangrado excesivo prolongado (≥10 días) mientras mudan el revestimiento existente, se ha demostrado que con el tiempo la tasa de amenorrea aumenta la atrofia del revestimiento. Así, el uso prolongado de este agente reduce la frecuencia de la menstruación, como así los síntomas relacionados con ella.
El acetato de medroxiprogesterona de depósito es ideal para las pacientes cuyos períodos menstruales representan un problema importante de higiene (por ej., niñas en desarrollo). “En nuestra experiencia,” dicen los autores, “las inyecciones se pueden dar a intervalos más breves para inducir rápidamente la atrofia del endometrio.”
En este escenario, el clínico puede dar una inyección cada 4 a 6 semanas, 2 a 3 dosis, para inducir la amenorrea y luego volver a administrar la dosis cada12 semanas. El principal riesgo al utilizar las inyecciones de acetato de medroxiprogesterona para inducir la amenorrea es que se puede perder hueso. Se ha demostrado que los usuarios de este método tienen un promedio más bajo de densidad mineral ósea y niveles más elevados de biomarcadores de la formación y reabsorción, comparados con los sujetos no usuarios. Sin embargo, estos cambios son similares a los observados en las mujeres que amamantan, son reversibles con el cese de la administración y no se asocian con mayor riesgo de fractura. En las adolescentes, el embarazo tiene riesgos óseos similares, con consecuencias a más largo plazo.
La medroxiprogesterona también puede estimular el apetito causando aumento de 10 a 20 kg de peso en las adolescentes y las mujeres que ya son obesas y tienen problemas con la regulación del apetito. Sin embargo, las mujeres delgadas no tienden a ganar peso.
Con esta información, el acetato de medroxiprogesterona de depósito parece ser una opción anticonceptiva con una buena relación costo beneficio que podría ser considerada, atendiendo a la situación clínica y la preferencia de cada paciente.
Parche anticonceptivo transdérmico
Ortho Evra es un parche transdérmico diseñado para liberar 0,02 mg de etinilestradiol y 0,150 mg de norelgestromina diarios. Por lo general, se aplica semanalmente durante 3 semanas, seguido de 1 semana sin parche, con el fin de inducir el flujo menstrual mensual por deprivación.
El uso extendido del parche para manipular la menstruación no está aprobado. En el único ensayo que evaluó el uso prolongado produjo amenorrea en el 12% de los usuarios, pero fueron comunes el sangrado no programado y el manchado.
Aunque hay alguna evidencia de que el uso a largo plazo del parche puede aumentar el riesgo de tromboembolismo venoso, se comprobó que el riesgo en las mujeres que usan el parche es similar al de las mujeres que usan anticonceptivos orales. Sin embargo, los niveles séricos de etinilestradiol son más elevados con el uso del parche semanal que con los anticonceptivos orales o el anillo vaginal con anticonceptivo, y como resultado, muchos médicos no se atreven a recomendar su uso continuado.
En espera de más datos sobre el perfil de seguridad de este anticonceptivo, la OMS recomienda que para los parches se utilicen los mismos criterios que para los anticonceptivos orales.
Anillo vaginal anticonceptivo
NuvaRing es un anillo vaginal anticonceptivo que libera una dosis diaria de 0,015 mg de etinilestradio y 0,12 mg de etonogestrel, Se coloca y se deja durante 21 días; luego se retira durante 7 días durante los cuales se produce el sangrado.
Se ha demostrado que la administración vaginal permite la dosificación baja y continua, lo que se traduce en concentraciones séricas más estables que con el parche o los anticonceptivos orales. En el único ensayo que comparó el anillo vaginal de permanencia extendida con el régimen tradicional de 28 días, se comprobó que el uso prorrogado dio lugar a menos días de sangrado que el uso mensual, pero con más manchado no programado.
Los efectos secundarios más comunes son el dolor de cabeza, la vaginitis y la leucorrea, pero no hay evidencia de cambios bacteriológicos o citológicos en el epitelio cérvicovaginal, incluso con el uso extendido.
Anticonceptivo implantable con etonogestrel
Implanon; se trata del implante de una barra de progestina sola disponible en Estados Unidos y otros países. Se coloca por vía subcutánea en la parte interior y superior del brazo y proporciona anticoncepción hasta 3 años de duración.
Implanon contiene 68 mg de la progestina etonogestrel, la cual va liberando lentamente con el tiempo. Inicialmente se administran 0,06-0,07 mg/día; 0,035-0,045 mg/día al final del primer año; 0,03-0,04 mg/día al final del segundo año y, 0,025-0,03 mg/día al final del tercer año.
La cantidad de sangrado vaginal asociado al uso del implante es generalmente escasa, pero el patrón tiende a ser impredecible. Por otra parte, debido a que la amenorrea aparece como efecto secundario en solo el 22% de las mujeres durante los 2 primeros años, el implante de progestina es un medio de supresión menstrual menos satisfactorio que los otros métodos aquí analizados.
• Beneficios de la Manipulación Menstrual
La manipulación menstrual tiene una serie de beneficios, tanto para la salud general como el estilo de vida. Para la mayoría de las mujeres, los anticonceptivos hormonales de acción prolongada tienen riesgos bajos y grandes beneficios para la salud. Las mujeres que toman anticonceptivos orales tienen menor riesgo de desarrollar osteoporosis, cáncer de ovario o de endometrio, cambios mamarios benignos o enfermedad inflamatoria pélvica.
El uso a largo plazo de un anticonceptivo oral también puede preservar la fertilidad reduciendo y retardando la incidencia de endometriosis, siendo eficaz en el tratamiento del acné vulgaris, que suele ser común entre los pacientes con síndrome de poliquistosis ovárica. Por otra parte, esta práctica se puede utilizar para reducir la pérdida sanguínea en general, una aplicación que es particularmente importante en las mujeres con diátesis hemorrágica, como la enfermedad de von Willebrand, en la cual es frecuente la menorragia.
La reducción de la menstruación también puede resultar más conveniente durante ocasiones especiales, como las vacaciones y las actividades deportivas. En concreto, puede ser útil para las mujeres que sirven en el ejército. En un estudio realizado por Schneider et al., en una cohorte de 83 cadetes femeninas informó un impacto importante de los síntomas premenstruales y menstruales sobre las actividades académicas, físicas y militares, como así las dificultades en la obtención, los cambios y el desecho de los materiales menstruales en un ambiente militar. Del mismo modo, la reducción de la frecuencia de las menstruaciones o la amenorrea puede desempeñar un papel importante en atletas de sexo femenino, quienes usan anticonceptivos orales para controlar los síntomas premenstruales, con el fin de proteger la salud ósea y para manipular el ciclo menstrual para mejorar su performance.
Las adolescentes son otro grupo que puede beneficiarse de la reducción o la ausencia de las menstruaciones, una vez que han llegado casi a su talla. Con la práctica de la supresión menstrual, las niñas pueden evitar la dismenorrea y los inconvenientes de la menstruación durante el día escolar, ya que su acceso a los analgésicos, las toallas higiénicas o los tampones y una muda de ropa puede estar limitado. Los clínicos que analizan junto con sus pacientes adolescentes los beneficios de los nuevos anticonceptivos que permiten programar las menstruaciones y reducir su frecuencia pueden mejorar la calidad de vida de estas jóvenes.
En una muchacha que recientemente ha tenido su menarca hay que tener cuidado en no iniciar los métodos hormonales demasiado pronto para no estrechar las epífisis de los huesos antes de tiempo y truncar la altura final. Después de la menarca, la mayoría de las niñas potencialmente aún pueden crecer unos 10 cm. más.
Por último, la manipulación menstrual también puede encontrar un nicho durante el desarrollo. Las mujeres con deterioro cognitivo y discapacidades físicas pueden tener dificultades para las prácticas de higiene relacionadas con las menstruaciones. Durante unos años, los anticonceptivos han sido utilizados para facilitar la higiene menstrual de las pacientes con epilepsia catamenial (o menstrual) o para abordar los problemas médicos en mujeres con retardo mental severo. En este contexto, se pueden obtener grandes beneficios de un agente que suprima la menstruación y proporcione anticoncepción, especialmente en las niñas y las mujeres en riesgo de abuso.
• Desventajas de la Manipulación Menstrual
Las tasas de eventos adversos y de interrupción de los regímenes de anticonceptivos orales extendidos y continuos son comparables a las de los tratamientos cíclicos, excepto las mayores tasas de sangrado.
En un ensayo de anticonceptivos orales continuos en más de 2.000 pacientes, 396 (18,5%) se retiraron del estudio como consecuencia del sangrado uterino molesto pero molesto. Sin embargo, aunque a menudo el sangrado ocurre durante los primeros meses del anticonceptivo oral extendido, por lo general disminuye con cada ciclo sucesivo de terapia y es comparable al sangrado que aparece en las pacientes que siguen el régimen de anticonceptivos orales por el cuarto ciclo extendido.
• Eficacia Anticonceptiva
La eficacia de la administración extendida y continua de los anticonceptivos orales es comparable a la de los regímenes cíclicos. Una de las razones puede ser una mejor adherencia a los regímenes vía oral: se ha demostrado que las mujeres que utilizan este régimen pierden menos toma de pastillas que las que siguen un régimen cíclico, especialmente durante la primera semana, al comenzar el envase.
Varios estudios han demostrado que algunas mujeres ovulan con el régimen oral 21/7, aun sin saltear ninguna pastillas o tomándolas fuera de horario, lo que las pone en mayor riesgo de embarazo. Estudios importantes que evaluaron la eficacia de un régimen extendido y continuo han mostrado una tasa de embarazo durante el período de estudio de 1 año comparable o inferior a la observada con los regímenes estándar.
Se debe recomendar a las parejas heterosexuales que usen condones, con el fin de reducir aún más el ya bajo porcentaje de fracaso y para evitar las enfermedades de transmisión sexual.
• Accesibilidad a la Manipulación Menstrual
Desde la primera prueba de un régimen oral extendido de anticonceptivos, las participantes han expresado una respuesta favorable en cuanto a la disminución de la frecuencia menstrual. Como rasgos favorables, en el estudio de Loudon et al, de 1.977, 6 pacientes con tratamiento extendido tuvieron períodos infrecuentes (82%), menos problemas menstruales (20%) y más fácil toma de la píldora (19%).
En 1.999, den Tonkelaar y Oddens encuestaron a 1.300 mujeres holandesas sobre la frecuencia menstrual preferida y hallaron que alrededor del 70% de ellas, de 15 a 49 años, prefirieron la frecuencia cada 3 meses y nunca. Una encuesta similar en Estados Unidos indicó que el 58% prefirió una frecuencia de sangrado cada 3 meses o nunca.
Mientras que generalmente las pacientes encuentran aceptable la manipulación menstrual, la aprobación clínica ha sido más variada. Loudon et al. informaron que "los médicos y las enfermeras de la clínica fueron menos entusiastas acerca de este régimen que los propios voluntarios."
El 90% de 222 médicos encuestados informó que nunca había prescrito un régimen extendido o continuo de anticonceptivos a adolescentes mientras que el 33% contestó que la prescripción para ciclos extendidos superó el 10% del total de la prescripción de anticonceptivos orales. Los autores sostienen que abundan los mitos y las percepciones erróneas acerca de la manipulación menstrual. Muchos clínicos no creen aconsejable el uso rutinario de un régimen extendido o continuo de anticonceptivos orales, a pesar de la falta de evidencia que avale su posición. Por lo tanto, se requiere más educación acerca de la práctica y los b beneficios de la manipulación menstrual.
• El Método Correcto para el Paciente Correcto
La manipulación y la supresión de la menstruación mediante el uso continuo o prolongado de anticonceptivos orales u otros medios puede tener una serie de ventajas para las mujeres, incluyendo la menor frecuencia de síndromes menstruales, la reducción del ausentismo laboral o escolar y la mayor satisfacción general.
Para las mujeres cuyo objetivo es reducir pero no necesariamente eliminar el sangrado mensual se ha propuesto el uso cíclico de anticonceptivos de estrógeno-progestina (en lugar de progestinas solas o el uso continuo de preparados hormonales combinados). Para las mujeres cuyo objetivo es la amenorrea, se recomiendan las inyecciones de acetato de medroxiprogesterona, los anticonceptivos orales continuos y el DIU con levonorgestrel, ya que todos son efectivos.
Aunque no se han hecho ensayos aleatorizados que comparen estos métodos, el acetato de medroxiprogesterona de depósito parece ser el de mayor tasa de amenorrea, mientras que el DIU con levonorgestrel es el más conveniente y parece estar asociado con menos efectos secundarios molestos que la inyección de progestina. Las pacientes que utilizan medroxiprogesterona de depósito deben vigilar su densidad ósea para detectar y prevenir la pérdida de hueso, mientras que los usuarios de los comprimidos de estrógeno-progestina, parche transdérmico o anillo vaginal no deben tener ninguna contraindicación para el uso de anticonceptivos con estrógenos.
Los médicos no deberían subestimar el riesgo de los anticonceptivos orales hormonales y otros métodos, sino más bien educarse a sí mismos de manera que puedan utilizar con seguridad la manipulación menstrual para satisfacer las necesidades individuales de las pacientes.
|
Contraindicaciones de los anticonceptivos orales estrógeno-progestina • Cáncer de mama u otro cáncer sensible a hormonas, actual o en el pasado. • Los tumores hepáticos, actuales o en el pasado, o enfermedad hepática. • Cualquier condición que predispone a las enfermedades trombóticas.Tromboflebitis o embolia pulmonar, actual o en el pasado. • Enfermedad cerebrovascular. • Enfermedad arterial coronaria. • Arritmia trombo valvular o trombogénica. • Hipercoagulopatías congénitas. Diabetes con enfermedad vascular. Hipertensión no controlada. Migrañas con síntomas neurológicos focales.umar y edad mayor de 35 años. Embarazo |
♦ Traducción: Dra. Marta Papponetti. Esp. Medicina Interna.